The French Pharmacopeia
By Eric Foxman, R.Ph., AAHP Secretary
A quick look at its structure and contents as well as two key considerations for potential adulteration and misbranding in the U.S. market.
Structure
The French Pharmacopeia (FP) is a publication of the National Agency for the Safety of Medicines and Health Products (ANSM), which is a public institution under the supervision of the French Ministry of Health. (This has an important consequence, to which we will return later.) ANSM’s mission is to ensure the safety of health products throughout their life cycle, from initial testing to post-market surveillance. Their mission specifically includes homeopathic products. [1]
The FP has a unique structure in comparison with other national pharmaceutical compendia: it contains monographs for both homeopathic and allopathic drug substances. This structure was subsequently adopted by the European Pharmacopeia (EP). Also in harmony with the EP, the homeopathic monographs appear in both English and French. (Other FP monographs and chapters are in French only.)
The FP and EP complement each other and, together, comprise the official compendia for France. Some FP monograph texts are slowly migrating into the EP. The EP analytical method requirements apply as referenced in the individual FP monographs; they can be supplemented by particular details in the FP when so labeled.
Like the HPUS, the FP is only available through online access.
Contents
Due to the harmonization between the national pharmacopeias and the recognized compendium for the entire European community, the FP contains very few general chapters, analytical methods, reagents or standards for materials/containers. The relevant chapters in the EP apply. Thus, manufacturers must be sure to reference both to ensure the appropriate information is at hand.
The FP officially recognizes very few specialized dosage forms. The short list includes emulsions, pills, pellets, suspensions, capsules. The EP contains an additional three dosage forms. In contrast, the HPUS Guidelines for Manufacturing Homeopathic Medicines lists 12 dosage forms.
As of 2107, the FP contained about 325 monographs of homeopathic drug substances. All other homeopathic preparations in France are non-official.[2]
Individual FP monographs have a slightly different format than those in the HPUS. Identification tests, additional tests, and assays for the starting material follow the definition of the monographed starting material. This definition includes information on which part of the plant is to be utilized. The second part of each monograph is titled, “Stock,” (see below) and gives identification tests, alcohol content, and dry residue limits, as well as assays for the prepared tincture.
IMPORTANT: There are many monographs in the HPUS and the FP that have the same name. However, there are about a dozen botanical monographs for which the HPUS and FP specify different plant parts; this difference is not identified in the name of the monographs. It is critically important to check with a supplier that uses the FP as their legal reference compendium to determine what plant part has been used and to compare that information with the HPUS. For the many hundreds of non-official homeopathic products in France, the need to double-check with the supplier is even more critical. If the foreign supplier uses a different plant part than that specified in the HPUS, the product will be considered adulterated and misbranded when sold in the United States. This is the first of two critical issues that may require access to, or understanding of, the FP.
The “Stock” and the First Attenuation
Initially, the first step for preparing homeopathic tinctures according to FP methods may seem the same as for those made according to HPUS. Both produce a product representing one part of botanical material in a total of 10 parts of finished tincture. However, unlike the HPUS, the resulting FP product is not yet a homeopathic drug product; it is an intermediate starting material from which homeopathic attenuations are made. This is reflected in the FP’s designation for this intermediate: it is the “stock” used to make all attenuations.
Put another way, even though an HPUS tincture and an FP stock are prepared in the same manner and concentration, their use is different. In the HPUS scheme, the tincture is the same as a 1X attenuation and is used to make the 2X attenuation. In the FP scheme, the stock is used to make the 1X attenuation (see Diagram 1). This is the critical consideration: what is labeled in France as a 1X is the same as what we label 2X in the United States!
The situation is further complicated when considering centesimal attenuations. A careful consideration of Diagram 2 reveals that centesimal attenuations sold in France are not equivalent in strength to HPUS centesimal attenuations and therefore cannot be interchanged.
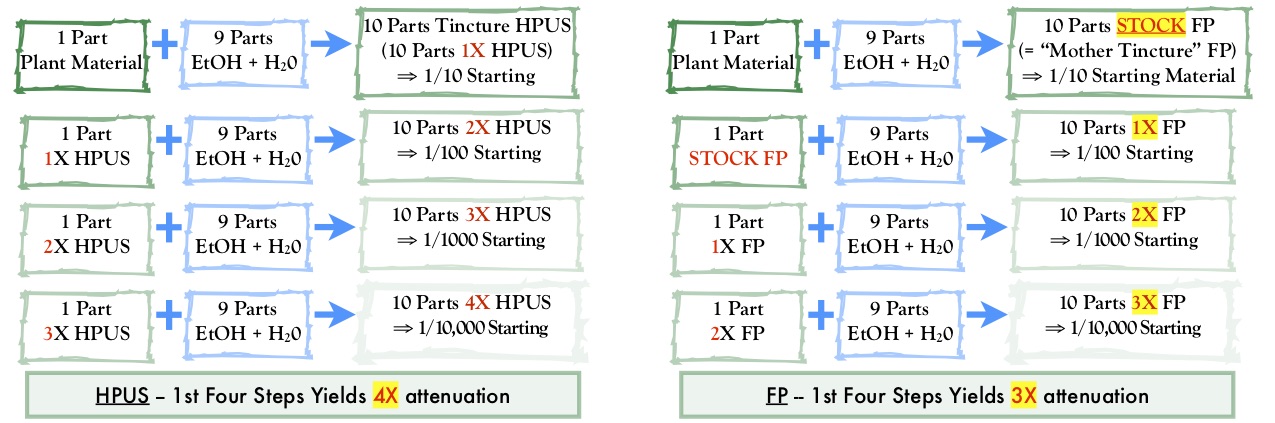
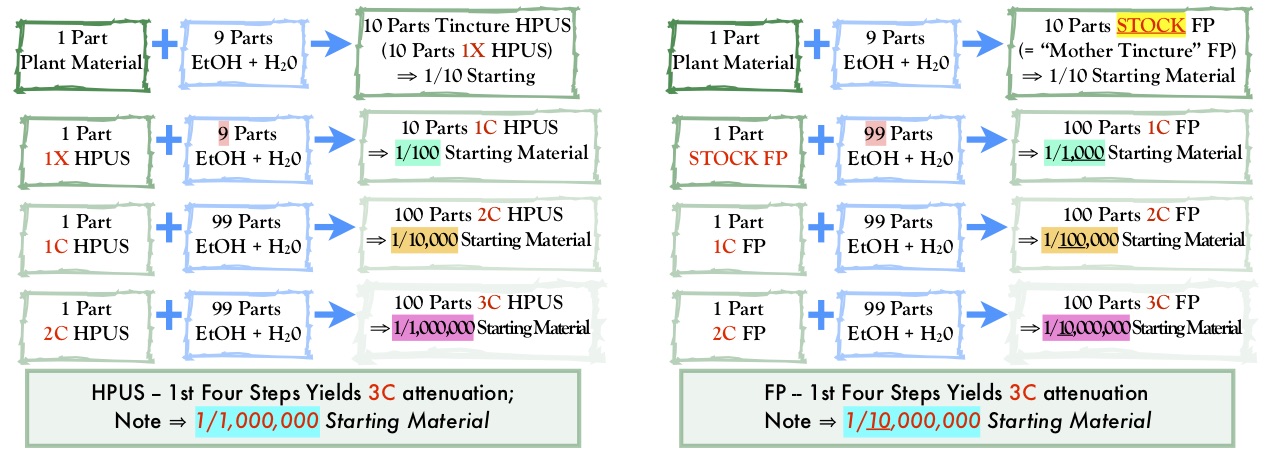
It is important to understand the following because of this difference in what the two pharmacopeias label as the first attenuation.
- A French company must be careful when manufacturing and labeling a product depending on whether it is for export to the United States or for sale in France.
- French homeopathic decimal attenuations that are not intentionally manufactured and labeled for export to the United States will be considered adulterated and misbranded when sold here.
- French homeopathic centesimal attenuations will be considered adulterated and misbranded if sold in the United States.*
- American homeopathic drug products exported to France must be manufactured and labeled for their appropriate attenuation level according to the FP, not simply prepared according to the HPUS criteria and exported.
(*Of course, if a homeopathic company is manufacturing products according to the HPUS for export to the United States, those are no longer ‘French’ homeopathic centesimal attenuations because they are not made according to the FP.)
Because FP is a publication of the French Ministry of Health, it is supported by tax revenues in France. As a consequence, access to the online document is free. It can be accessed here. The various texts are in French, with the exception of monographs for homeopathic preparations which are published in both French and English. Accessing the above link through an online translation program should make it possible to read all of the documents in English.
Notes
[2] “Non-official” means the substance has never been the subject of a monograph. This is in contrast to “unofficial” which means a previously official monograph has been deleted or discontinued.
