All
- All
- Articles
- Calendar
- Compliance
- Newsletter
- Position Statements
- Press Release
- Regulatory
- Uncategorized

Trump Nominates New FDA Commissioner
Senate Hearing Set for Nov. 20 By Pete Evich, AAHP Govt. Relations On Nov. 1, President Trump nominated Stephen Hahn, chief medical executive of...

4 Types of FDA Inspections & How to Prepare
Risk. It’s a hot word in the homeopathic industry right now. Even before the FDA issued its draft guidance, the agency used a risk-based approach...

Little-Known Facts About 3 Unique Starting Materials
By Mary Beth Watkins and Eric Foxman The array of substances used for homeopathic drug products is wide and varied. Many homeopathic starting materials...
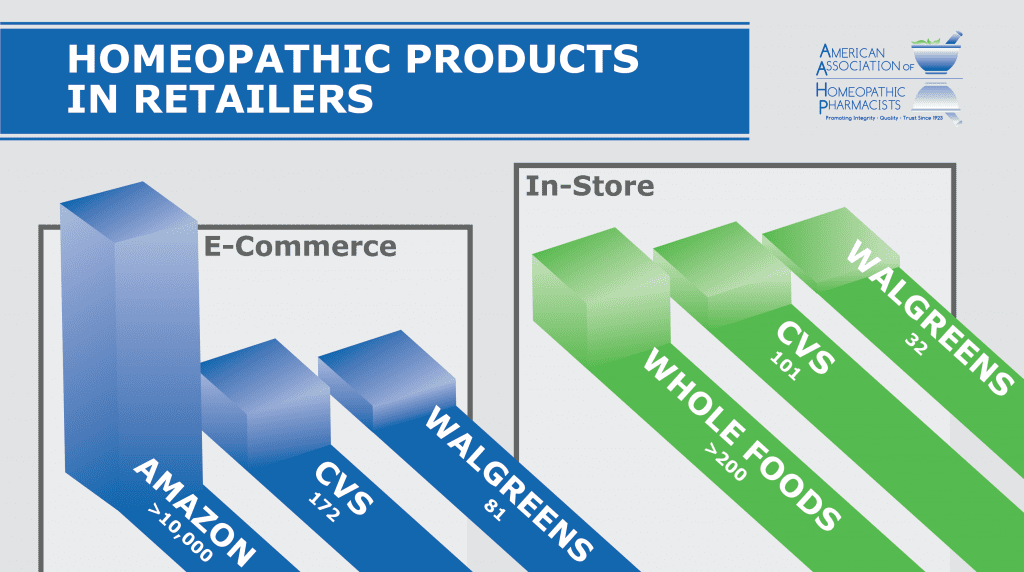
State of Homeopathic Medicines at Retail
By Mark Land, AAHP President Every year or so I survey my local CVS store for the number and type of homeopathic drug products...

Homeopathic Manufacturers Support FDA’s Revised Draft Guidance as Continuing Legal Status of Homeopathic Drugs
MILWAUKIE, Ore., Oct. 25, 2019 — Yesterday, the U.S. Food and Drug Administration (FDA) released a revised draft of a proposed guidance on homeopathic drugs...

Clearing the Air: Consider Slack-Fill
By Mark Land, AAHP President Although the term might not be familiar, you may have been fooled by “slack-fill” as a consumer of many...

The Amazon Deal You Never Heard Of
By Ray Petrick, VP of Sales, Boiron USA Amazon's accelerator program has existed for almost a year, and you've probably never heard of it....
