Stability Considerations of Homeopathic Drugs

By Mary Beth Watkins, AAHP vice president
July 1, 2017
The history of homeopathy has demonstrated that many homeopathic tinctures and low-potency dosage forms are remarkably stable. Archived homeopathic tinctures and potencies, when tested after years of storage, typically retain the essential and identifiable characteristics of the original tincture. While many botanical tinctures and chemical substances can be remarkably stable, everything is at risk of degradation over time. Finished products, formed into specific shapes or other characteristics, eventually succumb to degradation. Ensuring reliable stability characteristics is essential to maintaining efficacy and meaningful shelf lives.
The stability of active ingredients, be it tinctures and chemical or biological substances, can be subject to traditional analytical testing aimed at identifying their stability over time. Readers are encouraged to refer to stability references including ICH, USP, FDA, and HMPWG guidance when evaluating starting materials and low dilutions.
Stability assessment for homeopathic drugs across the industry includes a number of aspects of homeopathic products including the range of different dosage forms in the market and their various levels of complexity. The carriers used in each dosage form, such as lactose, magnesium stearate, sucrose, alcohol, glycerin, and emollients for topical application all provide specific advantages to each dosage form. Single remedies and complex mixtures both require a careful consideration of the correct assessment methods to be applied to each dosage form to determine product stability.
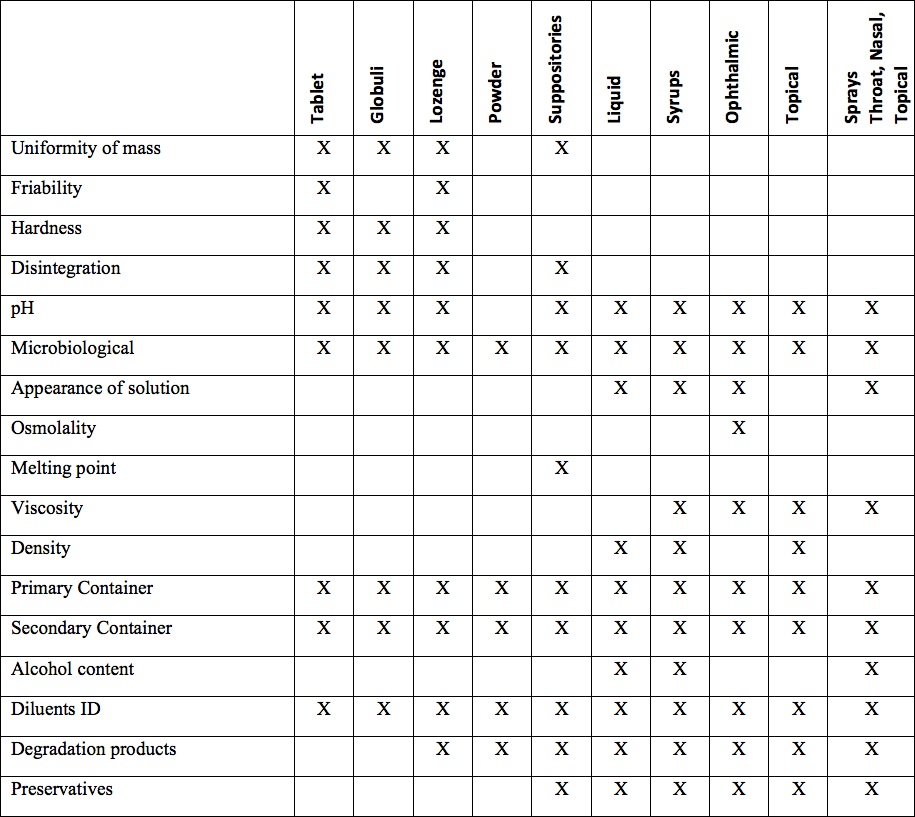
The homeopathic industry’s wide range of dosage forms include all of the following: globuli in several sizes; lactose-based tablets in a number of dosage sizes from 30 mg and 60 mg to 500 mg or more; and liquid dosage forms, include oral alcoholic liquids (20 percent to 80 percent ETOH) and glycerin based liquids, usually 45 percent. In addition, the market contains a wide number of less traditional homeopathic dosage forms, including nasal sprays, ophthalmics, and topical applications as creams, lotions, topical sprays, and oral throat spays. Homeopathic hard lozenges are also a significant product segment.
Each homeopathic dosage form is subject to its own set of stability requirements based on ingredients and manufacturing processes. Globuli are sucrose-based spherical pellets that are impregnated with homeopathic potencies and are dissolved in the mouth. This dosage form is remarkably stable if kept at room temperature and away from exposure to moisture and high humidity. Globuli should never be directly handled. The medication is coated on the outer layers of the sucrose pellet and handling can remove a large part of the medication.
One manufacturer has determined that their compressed tablets in sizes from 30 and 60 mg to 500 mg have shelf lives ranging from three to five years based on packaging which is well-sealed and moisture resistant. Packaging choices range from glass and plastic bottles, plastic tubes, and blister pack cards with or without outer cartons. Tablets can become fragile over time if adequate binder characteristics are missing. This can lead to friability and excessive chipping and in some cases, excessive powdering.
Homeopathic hard lozenges that are individually wrapped are then sealed in foil pouches or multilayer clear plastic bags. In another instance, a company’s stability program has shown their lozenges have a shelf life of three to five years based on the moisture barrier level of the packaging/bagging material.
According to yet a third set of assessments, a company’s liquid homeopathic medicines, containing
20 percent to 45 percent alcohol or higher and packaged in well-closed glass containers, are stable for two to five years and have been shown to remain stable for longer under well-controlled storage conditions. If a dropper bulb is used in the packaging, care must be taken to ensure that the bulb is of appropriate material and sufficient quality to resist the alcoholic contact from the bottle contents which can cause deterioration over time and contamination of the liquid.
The keys to adequate product stability are to select and formulate the carrier base carefully; test the unmedicated carrier prior to adding active ingredients for its stability; appropriate packaging components to retain product characteristics; and ensuring stability with a robust stability monitoring plan. Once the base carrier has been qualified, moving to full production of the solid dosage form is straightforward. Above is a chart of representative attributes to be tested by dosage form.
Keep it simple.