This Year’s AAHP Summit On Hold Until 2023

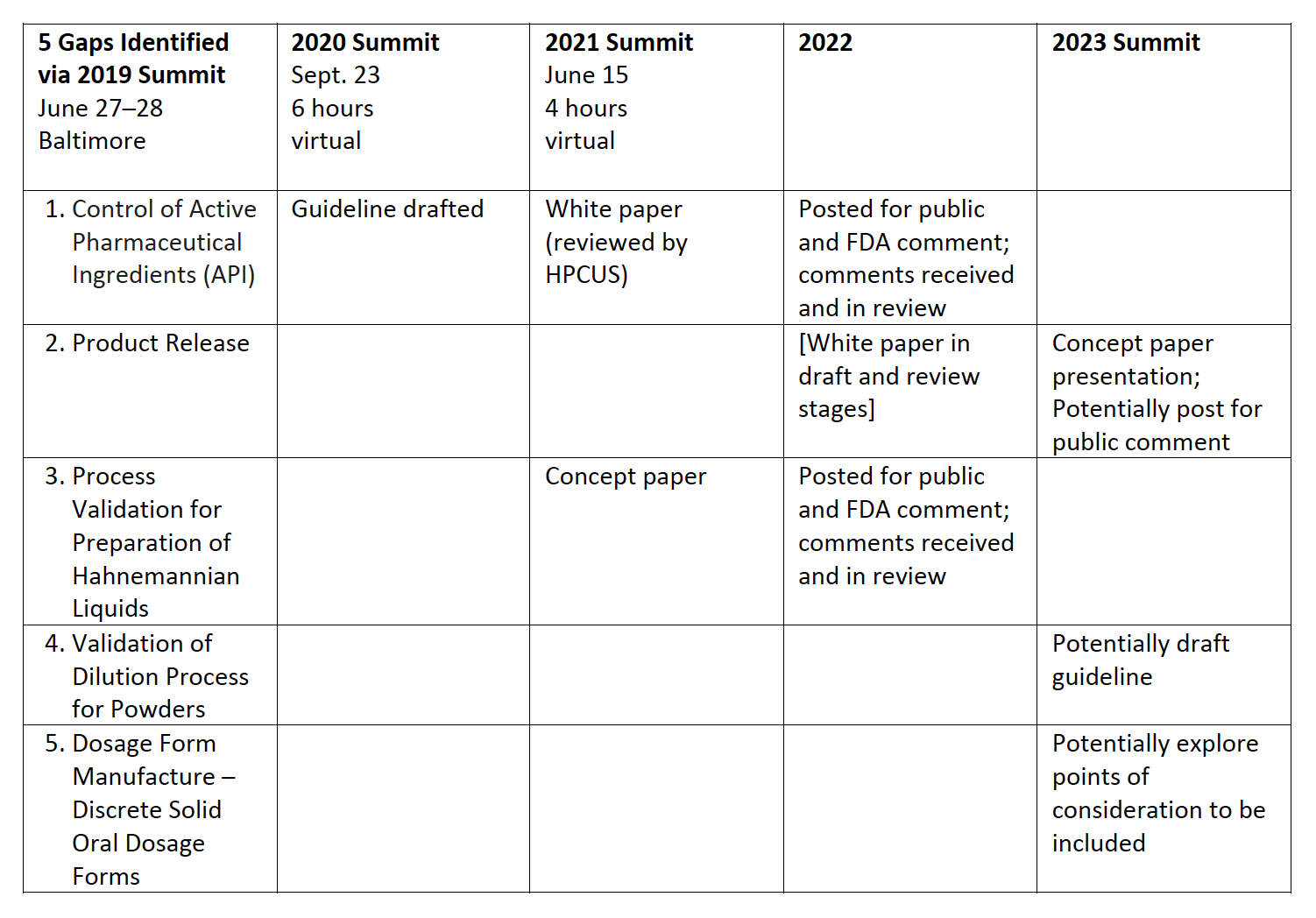
Now in its fourth year, the AAHP Summit series has gathered the industry for feedback while the HPCUS works through five compliance gaps that challenge manufacturers and FDA inspectors alike. The volunteers for this multiyear project are hard at work on the next phase, which upon completion will be the focus of the next Summit in 2023. Until then, there are some newsworthy teasers — including the receipt of FDA’s comments on two of the draft white papers.
Current Status
After presenting concept papers at the 2020 and 2021 Summits, incorporating industry feedback, and passing several industry member reviews, the Homeopathic Pharmacopeia Convention of the United States (HPCUS) posted two draft white papers on its website (October 2021 and January 2022). The HPCUS invited the FDA Compendial Operations and Standards Staff in the Office of Policy for Pharmaceutical Quality to review and comment on these white papers.
We are pleased to report the agency submitted meaningful comments on both the Starting Material white paper and the Hahnemannian Liquid Homeopathic Attenuations: Process Validation white paper. The HPCUS is currently reviewing FDA’s comments. The next step has already begun: review of the comments which are simple to incorporate (such as suggestions regarding nomenclature). In addition, the HPCUS has begun review of other comments which may be more far reaching in their meaning and consequences.
The AAHP appreciates FDA’s involvement in the HPCUS process. One byproduct of the project will be an overall strengthening of our industry’s communications with the agency. Pragmatically speaking, closing compliance gaps between 21 CFR cGMP requirements and the realities of bringing homeopathic drug products to market will help safeguard your business.
A Review of the Five “Gaps”
- Active Pharmaceutical Ingredients (API)
Guidelines are needed for starting materials, active drug substances, and intermediate and high dilutions in powder or liquid form. This will help to ensure the material received is compliant with the recipient’s expectations and with applicable regulatory requirements. The guideline must encompass topics listed under 21 CFR 211 Subpart E-Control of Components and Drug Product Containers and Closures. - Product Release
Guidelines are needed for appropriate laboratory testing of satisfactory conformance to final specifications for drug products, with consideration given to starting material diminution through sequential attenuation steps, resulting in very low concentration of any analyte in homeopathic products. The guideline must encompass the tests to be universally considered (identity, assay, impurities, and microbiological quality). - Validation of Dilution Process for Preparation of Hahnemannian Liquids
Guidelines are needed for validation of the dilution process used in manufacturing liquid homeopathic products, establishing validation procedures, potentially by groupings which would represent similar chemical structures and similar manufacturing conditions. The guideline must define the Hahnemannian liquid dilution process procedure steps, variables, and controls necessary to establish the process within a state of control. - Validation of Dilution Process for Powders
Guidelines are needed for validation of the dilution process used in manufacturing powdered homeopathic products. The guideline must define powder dilution process procedure steps, variables, and controls necessary to establish the process within a state of control. - Dosage Form Manufacture — Discrete Solid Oral Dosage Forms
Guidelines are needed to establish the blend uniformity (BU) and content uniformity (CU) requirements during the manufacturing of homeopathic drug products, including requirements for bulk hold study and powder segregation. The guideline must define procedures for BU and CU requirements during manufacturing homeopathic products will be produced.
If you’ve missed a Summit, now is the perfect time to catch up.
It’s not too late to review the previous presentations and join the discussion at the next Summit. Participation is encouraged for staff or consultants related to quality, safety, R&D, legal and regulatory, as well as senior company management. Feedback is encouraged on guidelines that may affect your company in the future.
- Summit 2019: This initial gathering of the industry in Baltimore (June 27–28) offered three tracks on quality, safety, and regulatory (for a total of nine presentations).
- Summit 2020: This 6-hour virtual event in September launched the multiyear project to develop HPUS guidelines for FDA compliance.
- Summit 2021: This 4-hour virtual event in June built upon the previous Summit. A white paper on Control of Active Pharmaceutical Ingredients (API) and a concept paper on Process Validation for Preparation of Hahnemannian Liquids were presented for feedback from the industry before summiting papers to FDA for the agency’s comments.
- Summit 2023: Watch for announcements!
Please email the AAHP Office if you are interested in viewing any of the previous presentations.
Don’t miss future HPCUS public comment opportunities for the next white papers. Sign up for the free HPUS email notifications for website updates.