New FDA Report on the State of Pharmaceutical Quality
By Mark Land, AAHP President
A quality drug is consistently safe, effective, and free of contamination or defects. Patients and consumers expect quality drugs with every dose they take. The homeopathic industry monitors the quality of its products, and so too does the U.S. Food and Drug Administration (FDA). Specifically, it is the Office of Pharmaceutical Quality (OPQ) in the Center for Drug Evaluation and Research (CDER) that is responsible for monitoring the quality of CDER-regulated drugs marketed in the United States.
Each year, OPQ releases its “State of Pharmaceutical Quality” report, a snapshot of the pharmaceutical manufacturing industry’s ability to deliver quality pharmaceutical products. Here is an overview of the report on 2019. We recommend taking a look to gauge common gap areas FDA may be assessing during inspections.
Why the Report Matters
OPQ uses the State of Pharmaceutical Quality to, among other things, inform regulatory decision-making and surveillance activities. OPQ also provides this information to internal FDA business partners to inform their operations. They are now providing this information publicly, so our external stakeholders can better understand the quality of the U.S. drug supply, and to better engage the pharmaceutical manufacturing industry in a commitment to quality.
What’s Measured?
FDA gauges this objective assessment using quality indicators based on available FDA drug product-specific and manufacturing site-specific data, including:
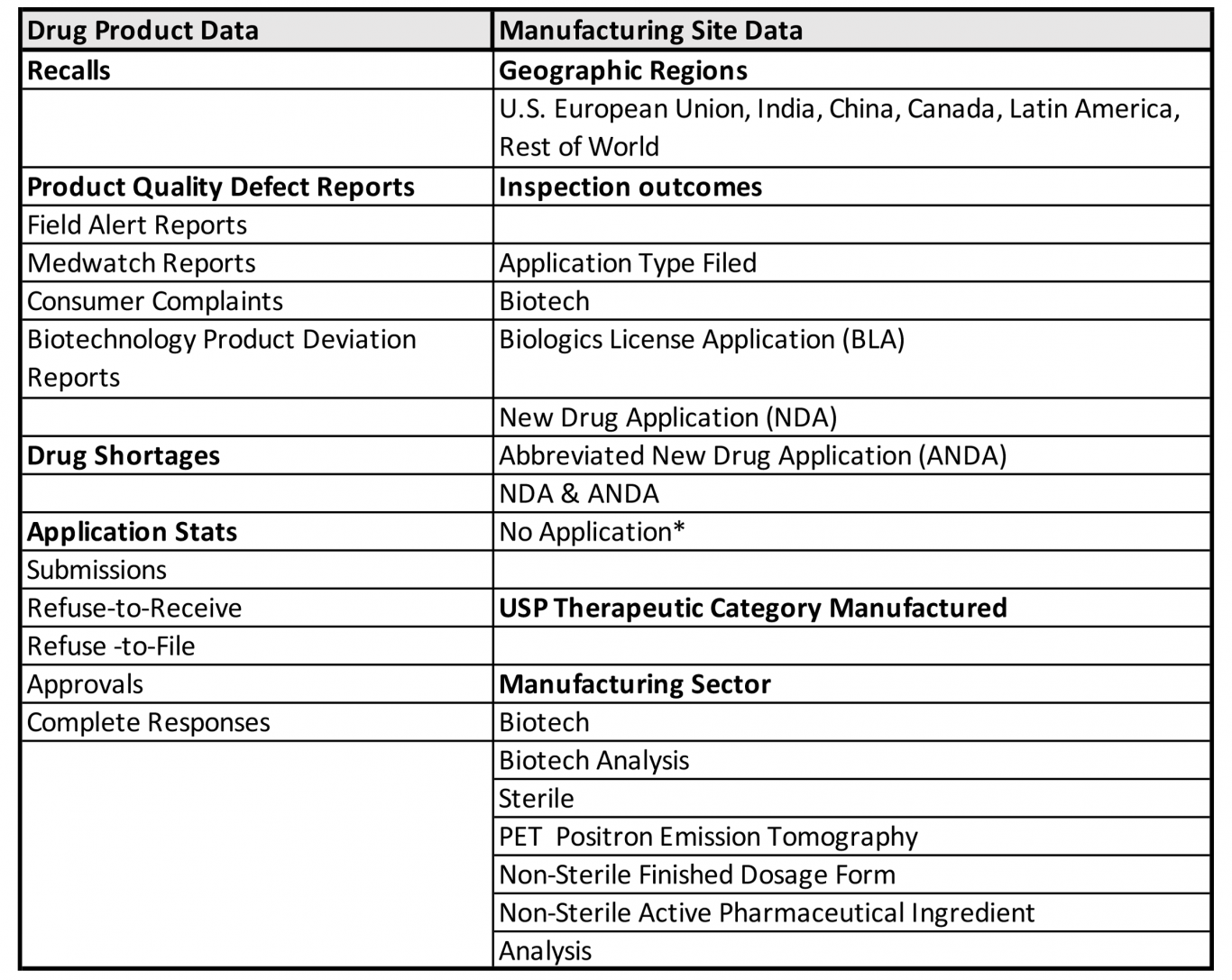
*No Application Sites are those sites not flagged as having any FDA-approved application products (e.g., OTC monograph, unapproved, homeopathics)
Highlights from the 2019 Report
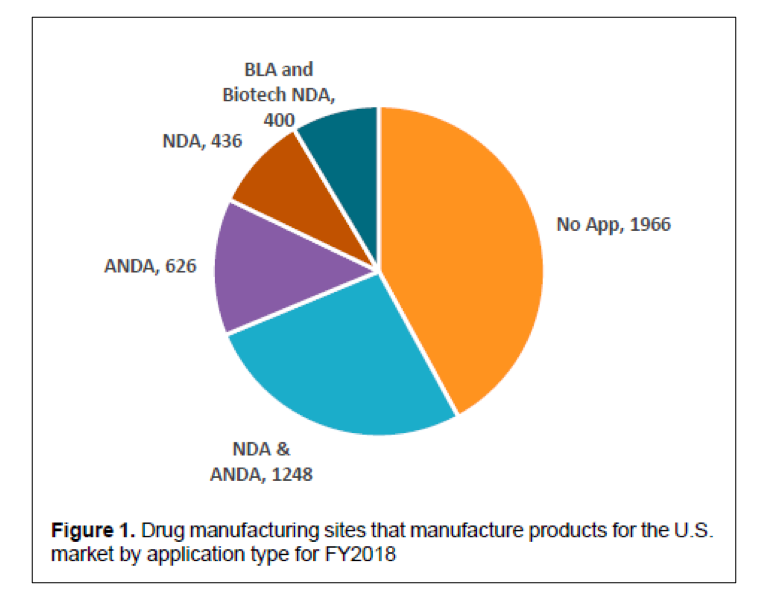
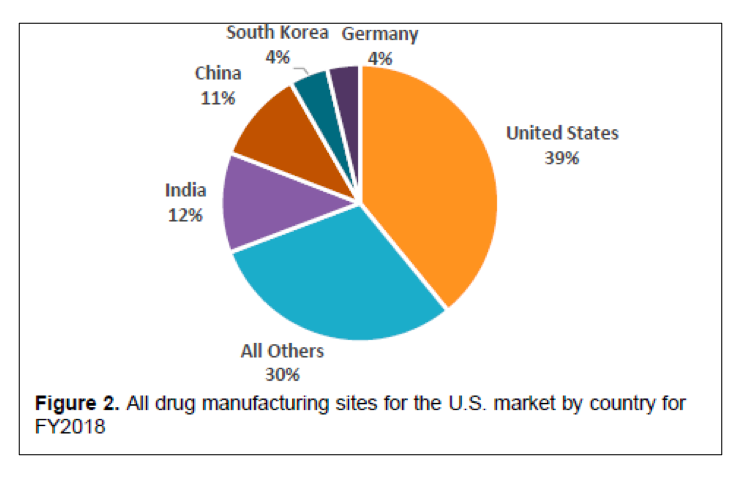
One of the outcomes of the State of Pharmaceutical Quality program is the site inspection score (RCS). The site inspection score provides one measure of a site’s compliance with CGMP regulations. The average score of all sites in FY 2019 was 7.4, not significantly different than FY 2018 (7.5). Still, there are some differences between geographic regions, application types, and manufacturing sectors (Figure 3). For example, the average scores for sites in the EU (7.7) and U.S. (7.6) are statistically higher than the global average, while the average score for sites in China (7.0), India (6.8), and Latin America (6.8) are statistically lower than the global average. All of these scores indicate an acceptable level of compliance to CGMPs on average. When considering application types, the No Application sector (6.7) significantly brings down the global average. Within this No Application sector, sites making homeopathic products (6.5) and OTC sterile products (6.2) scored lowest.
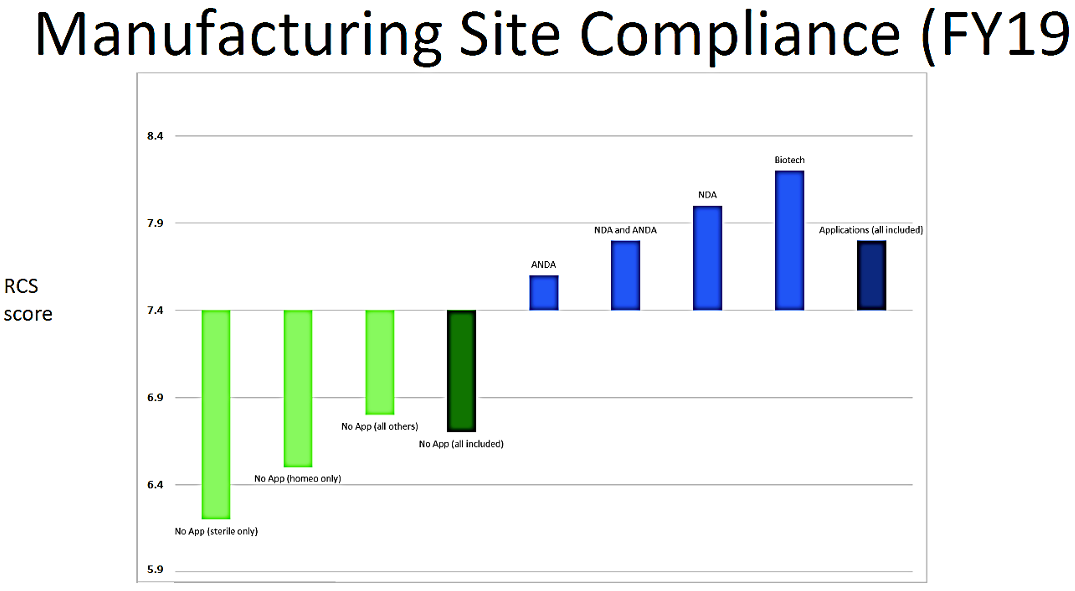
Figure 3. Site Inspection Score (FY 2019)
To a certain extent, homeopathic manufacturing site inspection scores continue to suffer from a disproportionate incidence of first inspections. Site inspection scores are an outcome of FDA’s quality metrics program. They are being made public as stated above. It is likely that the RCS will become important commercially as consumers, retailers, and other payers take notice.
References
- Report on the State of Pharmaceutical Quality, United States Food and Drug Administration, Center for Drug Evaluation and Research, Office of Pharmaceutical Quality. https://www.fda.gov/media/125001/download.
- Buhse, C., Quality Metrics and Quality Maturity, United States Food and Drug Administration, Center for Drug Evaluation and Research, Office of Pharmaceutical Quality. Presented at the Consumer Healthcare Products Association's Regulatory and Scientific Conference, Sept. 2, 2020.
- Land, M., Feb. 2015, FDA’s Quality Metrics Initiative – From Compliance to Performance, Network News, American Association of Homeopathic Pharmacists.
