COVID-19 CARES Act Aids Availability of OTC Products
On March 27, President Trump signed into law the Coronavirus Aid Relief and Economic Security (CARES) Act (HR 748). This landmark legislation provides financial relief for workers, small businesses, and healthcare professionals affected by the coronavirus. Within the massive $2 trillion stimulus package, though, there are two sections that may give Americans more access to over-the-counter (OTC) drugs. First, consumers will immediately benefit from purchasing OTCs with pre-tax Health Savings Accounts (HSA) and Flexible Spending Accounts (FSA). Second, consumers may later benefit by innovative products that will be brought to market by reforming the FDA approval process.
Restoration of OTC Eligibility under HSA/FSA Accounts
OTC drugs, including homeopathic OTC products, can again be purchased using tax-preferred funds by the nearly 60 million Americans enrolled in FSAs and HSAs.
OTCs were first eligible under tax-preferred FSAs and HSAs in 2003 but, seven years later in 2010, the Affordable Care Act required a prescription for medicines purchased through these funds. Confused consumers sought prescriptions for non-prescription products, thus creating unnecessary strain on the U.S. healthcare system.
The current reversal unburdens the system and is especially useful now during the pandemic and unemployment crisis. The legislation encourages consumers to depend more on OTC products for relief of self-treatable symptoms rather than unnecessarily physically interacting with overwhelmed and at-risk healthcare professionals. The measure will also help reduce the spread of coronavirus.
Furthermore, the law is retroactive. Any qualified OTC purchase since January 1, 2020 can be reimbursed through the pre-tax funds, which may be helpful for the 22 million Americans suddenly unemployed. Technically speaking, the CARES Act amends the Internal Revenue Code at 26 USC:
- § 105 106(f) Flexible Spending Arrangements
- § 223 (d)(2)(A) & (D) Health Savings Accounts
Nothing in the law or IRS circular prohibits homeopathic drug products from qualifying medical expenses. Retailers will require manufacturers to register OTC products on certain clearinghouse lists to facilitate recognition.
OTC Monograph Reform
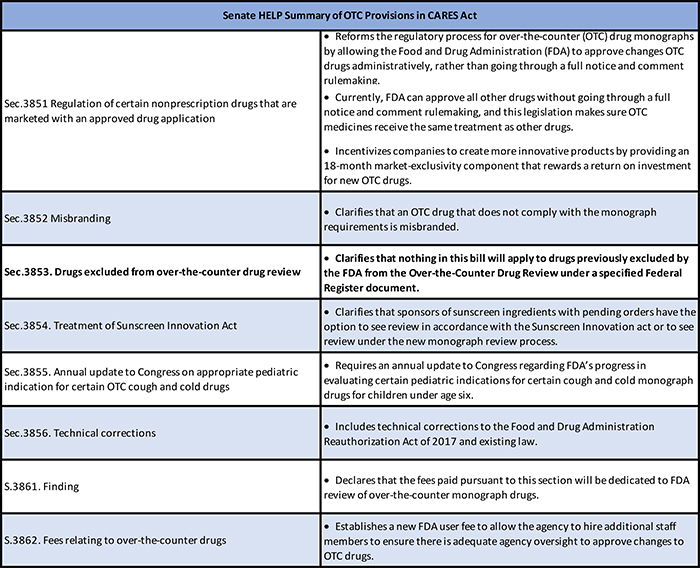
The second way the CARES Act may provide Americans more access to OTCs is through a section that updates how FDA has regulated the majority of these medicines for the past 48 years. FDA uses the OTC monograph system to review the safety and efficacy of ingredients, doses, formulations, and labeling for non-prescription drugs.
Over the years, this principly-sound system became outdated, cumbersome, and in desperate need of modernization. Updates to existing monographs based on new science currently could take years due to the multi-layered rulemaking process, lack of funding and lack of FDA staff. These difficulties in updating product labels with new safety information created a backlog of monographs. The new provisions in the CARES Act are essentially identical to the legislation passed overwhelmingly by the U.S. Senate, 91-2, this past December. Read more about the benefits of Monograph Reform for conventional OTCs through the Consumer Healthcare Products Association, who has campaigned this legislation for years.
In a March 30 positive statement, FDA Commissioner Stephen Hahn specifically highlighted the monograph reform portion of the bill. “[It] reforms and modernizes the way certain over-the-counter (OTC) drugs are regulated in the United States — a landmark step that will have an impact lasting long after the current public health emergency. The law grants FDA transformative, new authorities that will meaningfully advance the agency’s efforts to modernize the OTC drug development and review process to help advance innovative, safe and effective options for consumers and secure a robust OTC marketplace. FDA is committed to using these new tools to promote innovation and improve the safety and effectiveness of OTC monograph drugs — including products like hand sanitizers and acetaminophen, which are so critical to the current public health emergency.”
The FDA’s statement references that the ACT applies to “certain” OTCs. Of note to homeopathic manufacturers is SEC. 3853. Drugs Excluded from the Over-the-Counter Review:
(a) IN GENERAL.—Nothing in this Act (or the amendments made by this Act) shall apply to any non-prescription drug (as defined in section 505G(q) of the Federal Food, Drug, and Cosmetic Act, as added by section 3851 of this subtitle) which was excluded by the Food and Drug Administration from the Over-the-Counter Drug Review in accordance with the paragraph numbered 25 on page 9466 of volume 37 of the Federal Register, published on May 11, 1972.
(b) RULE OF CONSTRUCTION.—Nothing in this section shall be construed to preclude or limit the applicability of any other provision of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 301 et seq.).
This language confirms the exclusion of homeopathic products from OTC monograph reform/user fee amendment. This is important because the monograph reform provisions give FDA the authority to regulate by administrative orders, which might have enabled the agency to regulate homeopathic drugs as a group, something it cannot do now.
In total, FDA will receive an additional $80 million in funding to continue the Agency’s COVID-19 response efforts, including the development of medical countermeasures and vaccines, promoting the advanced manufacturing of medical products and monitoring of the medical product supply chain.
Senators Lamar Alexander (R-Tennessee), Bob Casey (D-Pennsylvania), and Patty Murry (D-Washington) were instrumental in dislodging OTC Monograph Reform and the Medical Expense legislation frozen by partisanship and ensuring passage in the house by including Sunscreen Reform. All three are leaders in the Senate Health, Education, Labor, and Pension (HELP) Committee. AAHP thanks the senators for their inspired parliamentarianism.
AAHP also thanks all those who put themselves at risk to help the public — from the integrative healthcare professionals in the forefront to staff fulfilling the orders at AAHP member companies.
