FDA Office of Surveillance and Epidemiology
By Mark Land, AAHP President
April 1, 2017
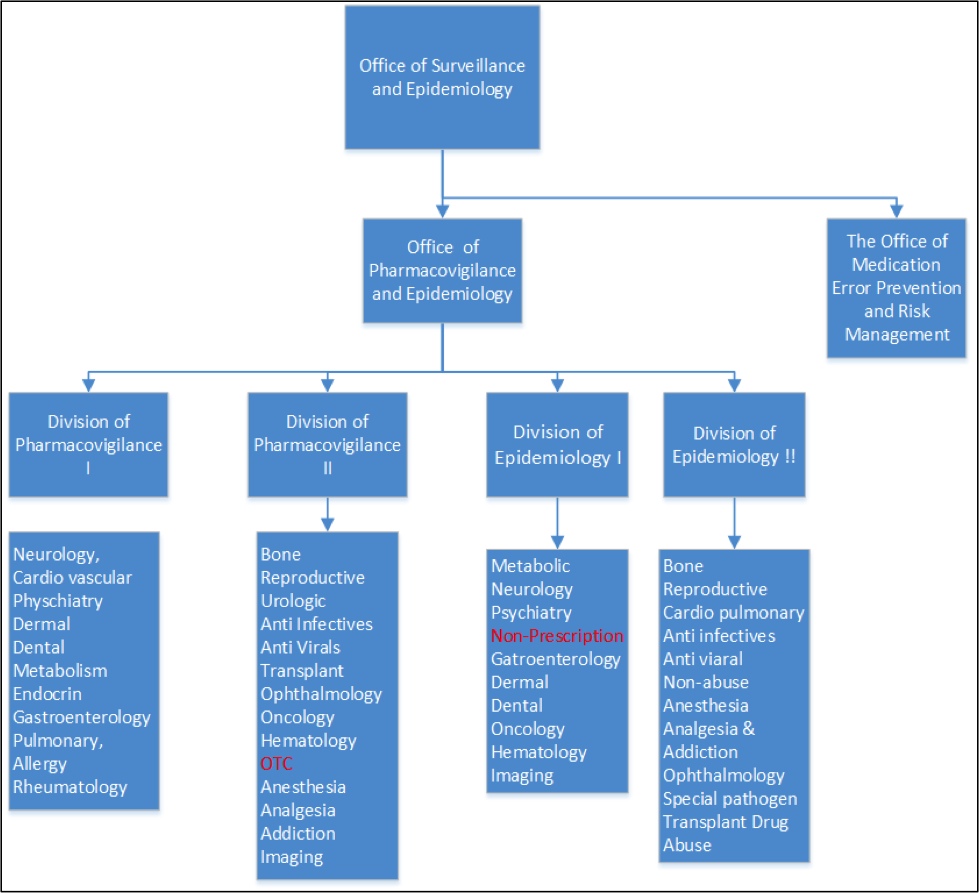 FDA’s Office of Surveillance and Epidemiology (OSE) is positioned within the Center for Drug Evaluation and Research (CDER). OSE evaluates the safety profiles of drugs available to American consumers using a variety of tools and disciplines throughout the life cycle of drugs. OSE maintains a system of postmarketing surveillance and risk assessment programs to identify adverse events that did not appear during the drug development process. FDA staff learn about adverse events through required reporting by companies and through voluntary reports submitted to FDA’s MedWatch program, which totals more than one million reports per year. Staff in the Office of Surveillance and Epidemiology use this information to identify drug safety concerns and recommend actions to improve product safety and protect the public health.
FDA’s Office of Surveillance and Epidemiology (OSE) is positioned within the Center for Drug Evaluation and Research (CDER). OSE evaluates the safety profiles of drugs available to American consumers using a variety of tools and disciplines throughout the life cycle of drugs. OSE maintains a system of postmarketing surveillance and risk assessment programs to identify adverse events that did not appear during the drug development process. FDA staff learn about adverse events through required reporting by companies and through voluntary reports submitted to FDA’s MedWatch program, which totals more than one million reports per year. Staff in the Office of Surveillance and Epidemiology use this information to identify drug safety concerns and recommend actions to improve product safety and protect the public health.
Office of Pharmacovigilance and Epidemiology
Division of Pharmacovigilance
Medical Officers in the Divisions of Pharmacovigilance I and II detect safety signals and assess safety-related issues for all marketed drug and therapeutic biologic products. They use a variety of surveillance tools including adverse event report data, published scientific literature, and preclinical, clinical, and pharmacologic knowledge of the products to provide scientific and clinical evaluation leading to various regulatory actions and communications for safe use of the marketed products.
Division of Epidemiology
Epidemiologists in the Division of Epidemiology conduct active drug safety surveillance using Sentinel’s ARIA system (Active Risk Identification and Analysis), and review drug safety-related epidemiologic study protocols and study reports that are required of manufacturers as post marketing requirements (PMRs) and commitments. They evaluate safety signals that arise by putting them into the context of drug use, the existing body of evidence in the scientific literature, and by mounting FDA-sponsored epidemiologic studies as needed to quantify and characterize drug safety risks detected through spontaneous reports or through systematic review of the scientific literature. In addition, the drug utilization team provides denominator data (exposure rate), for modeling drug risk based on usage patterns; and for calculating patient-based reporting rates. They also provide data that aid in increasing the FDA’s ability to request regulatory impact studies such as those authorized under Best Pharmaceuticals for Children Act (BPCA) to better understand the usefulness of drug labeling to clinicians.
Drug Utilization Analysis Staff
Drug Use Analysts manage and analyze pharmaceutical sales and health care data to describe and characterize drug utilization levels and treatment patterns in the U.S. to provide context for drug safety issues in support of regulatory decision-making in FDA/CDER.
The Office of Medication Error Prevention and Risk Management
The Division of Medication Error Prevention and Analysis is primarily responsible for the premarket review of proposed proprietary medication names, labels/labeling, packaging, and Human Factor Studies to reduce the potential for medication errors for CDER-regulated products.
The Division of Risk Management serves as the focal point for risk management activities in CDER. DRISK provides risk management expertise on development and implementation of programs and initiatives to support the Center’s policies related to Risk Evaluation and Mitigation Strategies (REMS) authorities under the Food and Drug Administration Amendments Act (FDAAA) of 2007.
The processes of these offices mimic the work of Pharmacovigilance and Epidemiology departments in pharmaceutical companies in general. Simply put, Pharmacovigilance in large part collects and analyzes adverse event reports and Epidemiology places that information into population level context. Pharmacovigilance is in interested in the authenticity of signals and trends from individual reports and Epidemiology determines their importance within the exposed population. Despite the perceived safety of homeopathic drug products pharmacovigilance and eventually epidemiology are important and necessary.
Notes:
- Sentinel is an active surveillance system sponsored by the U.S. Food and Drug Administration to monitor the safety of regulated medical products using pre-existing electronic healthcare data from multiple sources. The Sentinel System is part of the FDA’s Sentinel Initative, a long term effort to improve the FDA’s ability to identify and assess medical products safety issues.
- Active Risk Identification and Analysis (ARIA) is FDA’s active post-market risk identification and analysis system, which is comprised of pre-defined, parameterized, re-usable routine querying tools that enable safety surveillance in Sentinel. In contrast to a traditional pharmacoepidemiology study, there is no protocol and no customized programming. ARIA fulfills the FDA’s Amendments Act mandate to conduct drug/medical product safety surveillance.
References:
- Office of Surveillance and Epidemiology Organizational Chart. U.S. Food and Drug Administration. Available at https://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/UCM485202.pdf.
- Office of Surveillance and Epidemiology Divisions. U.S. Food and Drug Administration. Available at https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm169536.htm.
- Gerald J. Dal Pan, MD, MHS. CDER Office of Surveillance and Epidemiology: 2016 Update; December 14, 2016. Available at https://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/UCM533535.pdf.
- About the Sentinel System. Available at https://www.sentinelinitiative.org/sentinel/about.
- Active Risk Identification and Analysis. Available at https://www.sentinelinitiative.org/active-risk-identification-and-analysis-aria.
