Document Management Systems
 It is often said that the pharmaceutical industry produces two products: healthcare products and the paper that goes along with it. During the past 20 years, we have seen a transition from paper-based format to the production of electronic-based information that provides a rich foundation of presentation options. The pharmaceutical industry was reluctant to adopt electronic systems owing to the uncertainty surrounding electronic records compliance. Now, in today’s globalized environment, compliance concerns have transitioned to include data integrity.
It is often said that the pharmaceutical industry produces two products: healthcare products and the paper that goes along with it. During the past 20 years, we have seen a transition from paper-based format to the production of electronic-based information that provides a rich foundation of presentation options. The pharmaceutical industry was reluctant to adopt electronic systems owing to the uncertainty surrounding electronic records compliance. Now, in today’s globalized environment, compliance concerns have transitioned to include data integrity.
Compliance concerns should not limit your investigation and/or implementation of electronic records or records management systems. Recent FDA inspections demonstrated to me that electronic systems can reduce the inspection cycle by several days.
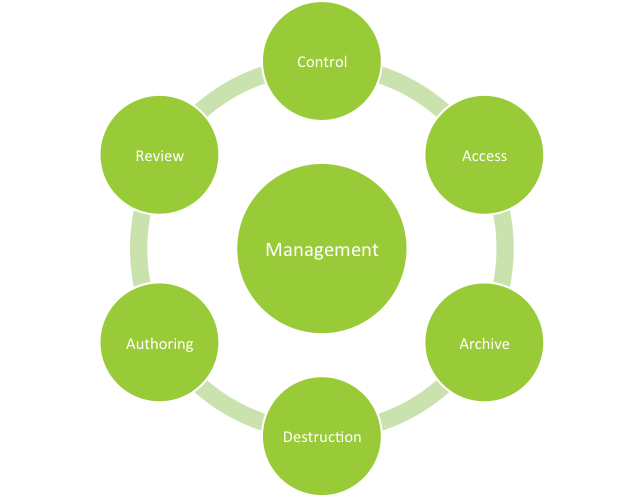
For those of you not familiar with document management systems for the pharmaceutical industry, they are impartial partners in the development, review, control, archiving and retrieval of regulated documents. Document management systems enhance regulatory compliance through: authentication, access management, revision scheduling and archiving to name a few of their benefits.
Imagine full electronic traceability throughout the document life cycle, beginning with authoring through the review archiving and destruction processes. Automated document management systems provide electronic distribution of documents across departments or facilities with confirmation of receipt in support of common quality management systems. Document management systems are not inexpensive and require effort to establish within your organization but the results justify the investment.
