All
- All
- Articles
- Calendar
- Compliance
- Newsletter
- Position Statements
- Press Release
- Regulatory
- Uncategorized

How to Stop Bad Inspections: It Starts with Staff
By Mark Land, AAHP President At AAHP, we firmly believe that compliance with quality and safety regulations is crucial for all homeopathic manufacturers. To...
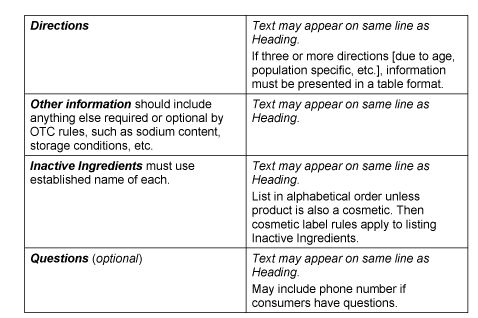
Anatomy of a Compliant Drug Facts Panel
By Eric L. Foxman, R.Ph. In my May 2020 article, The Anatomy of a Compliant Homeopathic Label, I covered: The importance of considering the...

The Anatomy of a Compliant Homeopathic Label
By Eric L. Foxman, R.Ph., AAHP Secretary Before examining the details of a compliant homeopathic drug product label, it is important to consider labeling in...

Is the Homeopathic Industry Underinvesting in Quality Systems?
By Mark Land, AAHP President I’ve been pondering this question for some time, ever since preparing for my presentation on FDA enforcement trends during...

Retail in the COVID-19 Pandemic
By Ray Petrick, VP Sales, Boiron USA The COVID-19 pandemic will likely be very painful for many legacy retailers such as Kohl’s, J.C. Penney,...
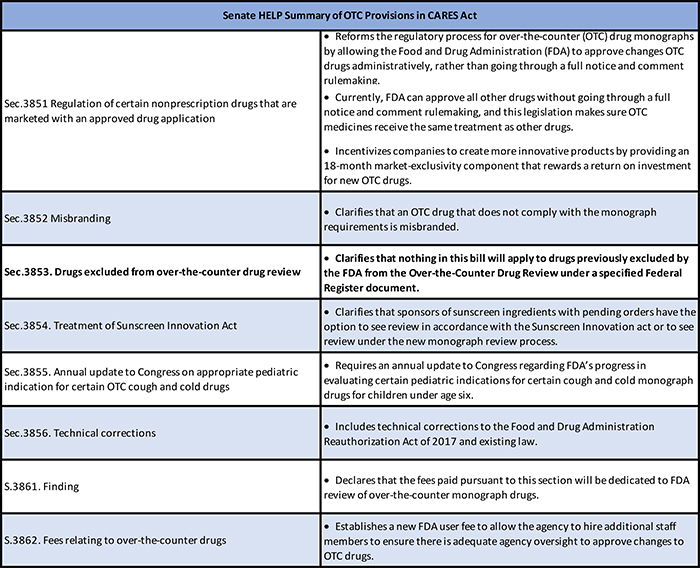
COVID-19 CARES Act Aids Availability of OTC Products
On March 27, President Trump signed into law the Coronavirus Aid Relief and Economic Security (CARES) Act (HR 748). This landmark legislation provides financial relief...
FDA’s Compendial Operations and Standards Branch (COSB)
FDA’s Compendial Operations and Standards Branch (COSB) a branch within FDA’s Office of Pharmaceutical Quality (OPQ) is responsible for the management of agency staff selection...
