All
- All
- Articles
- Calendar
- Compliance
- Newsletter
- Position Statements
- Press Release
- Regulatory
- Uncategorized

Belladonna: A Clinical Snapshot
By Todd Hoover, MD September 1, 2017 The original proving of belladonna was conducted by Dr. Samuel Hahnemann, who found it to become a commonly...

How is Homeopathic Belladonna Made?
By Mark Land, AAHP president September 1, 2017 Belladonna, more commonly known as deadly nightshade, Atropa belladonna, devil's cherries, devil's herb, divale, dwale, dwayberry, great morel,...

What’s New at FDA?
By Mark Land, AAHP president September 1, 2017 I took a course several years ago titled Regulatory Intelligence. The first thing the professor said was,...

When Anti-Homeopathy Skeptics Strike
By Deborah Kelly and Alissa Gould, PR Managers, Boiron USA September 1, 2017 It’s typical for skeptics to fire away at anyone who posts or...

The Road to OTC Monograph Reform
By Barbara A. Kochanowski, senior vice president of regulatory and scientific affairs Consumer Healthcare Products Association August 1, 2017 For the past three years, the...

Changes are Coming to Product Labels
By Alvin J. Lorman, AAHP counsel August 1, 2017 Changes are coming to your products’ labels. Not only are there changes to the Homeopathic Pharmacopeia...
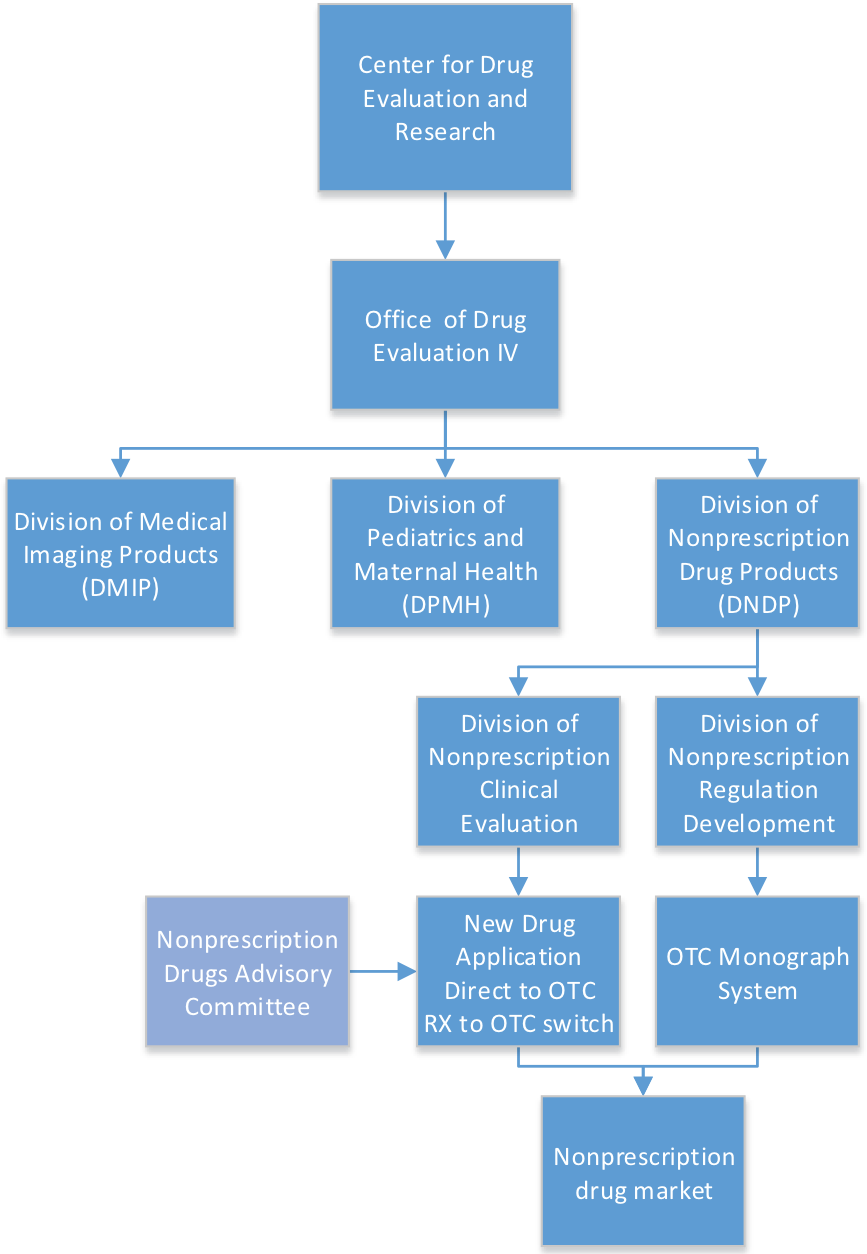
FDA’s Division of Nonprescription Drug Products
By Mark Land, AAHP president August 1, 2017 The development and regulation of over-the-counter (OTC) drug products is the responsibility of the Division of Nonprescription...
