All
- All
- Articles
- Calendar
- Compliance
- Newsletter
- Position Statements
- Press Release
- Regulatory
- Uncategorized

Congress Aiming to Consider User Fee Legislation in July
By Pete Evich, AAHP government relations July 1, 2017 Bills (S 934, HR 2430) that would renew the Food and Drug Administration’s (FDA) ability to...
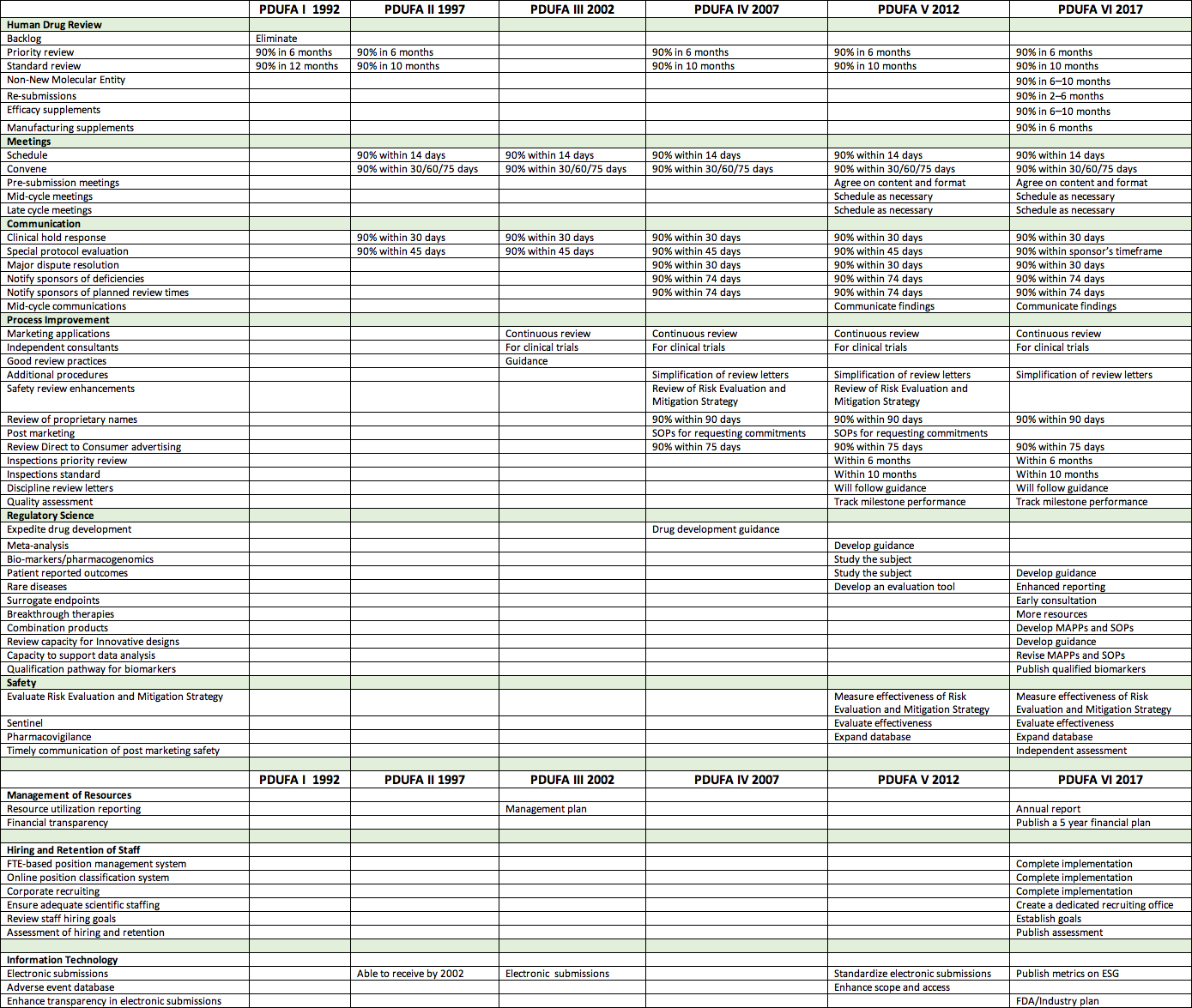
PDUFA after 25 Years
By Mark Land, AAHP president July 1, 2017 The Prescription Drug User Fee Authorization (PDUFA) first enacted in 1992 was the third major piece of...

Stability Considerations of Homeopathic Drugs
By Mary Beth Watkins, AAHP vice president July 1, 2017 The history of homeopathy has demonstrated that many homeopathic tinctures and low-potency dosage forms are...

Communications Best Practices: Anticipating Questions
By Alissa Gould, public relations manager, Boiron USA July 1, 2017 Since April 2017, AAHP has offered a series of articles on good communication practices...

The Homeopathic Pharmacopoeia of the United States
By Mark Land, AAHP president June 1, 2017 The Homeopathic Pharmacopoeia of the United States (HPUS) is the legal basis for marketing homeopathic drug products...

2017 Updates in the Homeopathic Pharmacopeia
By Eric L. Foxman, R.Ph., senior scientist, HPCUS June 1, 2017 Before proceeding to review the major areas of updates in the HPUS, two specific...

Delivering Key Messages
By Alissa Gould, public relations manager, Boiron USA June 1, 2017 Building on April and May 2017 articles on preparing for important communications and developing...

