All
- All
- Articles
- Calendar
- Compliance
- Newsletter
- Position Statements
- Press Release
- Regulatory
- Uncategorized

Disclaimers in Advertising and Labeling: What Do They Do? Who Does What?
By Mark Land, AAHP President The FTC has long recognized that marketing claims may include additional explanatory information to prevent the claims from being misleading....
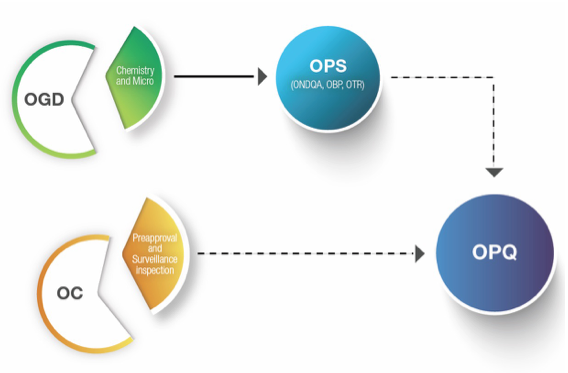
United States Food and Drug Administration Office of Pharmaceutical Quality
By Mark Land, AAHP President May 1, 2017 The Office of Pharmaceutical Quality (OPQ), created in Jan. 2015, enhances the U.S. Food and Drug Administration...

AAHP Recognized as Voice of Homeopathy on Capitol Hill
MILWAUKIE, Ore., Apr. 10, 2017 — The American Association of Homeopathic Pharmacists’ (AAHP) Legal & Regulatory Committee recently received the 2017 Henry N. Williams Professional...

Be Prepared: Media Basics for the Non-PR Expert
By Alissa Gould, public relations manager, Boiron USA April 1, 2017 As more people discover the benefits of homeopathic medicines, our industry may become a...
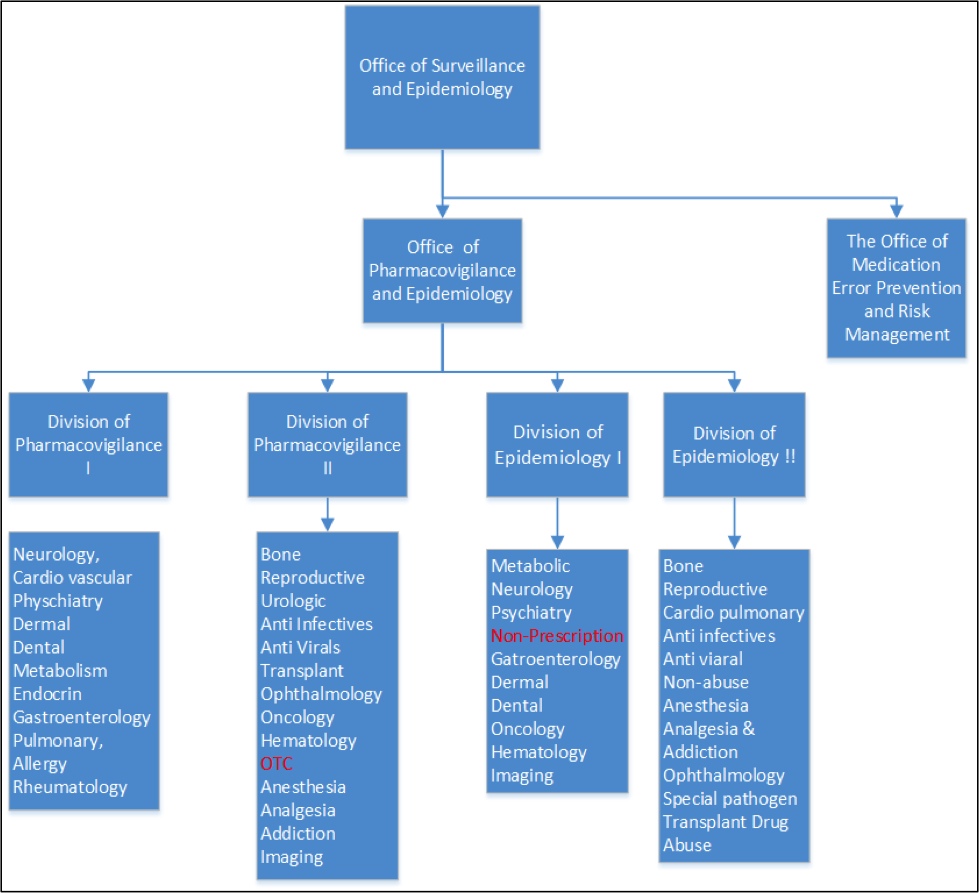
FDA Office of Surveillance and Epidemiology
By Mark Land, AAHP President April 1, 2017 FDA’s Office of Surveillance and Epidemiology (OSE) is positioned within the Center for Drug Evaluation and Research...

Analytical Methods
By Mark Land, AAHP President April 1, 2017 When Samuel Hahnemann founded homeopathy at the end of the 18th century, he couldn’t possibly have fathomed...

The State of Technology in Homeopathic Medicine
By Jim Duquesnel, VP-R&D, Hyland's, Inc. April 1, 2017 “Be careful what you ask for!” This phrase surfaces more often than I would like in...
