All
- All
- Articles
- Calendar
- Compliance
- Newsletter
- Position Statements
- Press Release
- Regulatory
- Uncategorized

Homeopathic Manufacturers Honor Sen. Barbara Ann Mikulski for Career-Long Support of Complementary Health Care
MILWAUKIE, Ore., Oct. 4, 2016 — Senator Barbara Ann Mikulski (D-Md.) recently received a Legislative Excellence Award from the American Association of Homeopathic Pharmacists (AAHP),...

FTC Holds Workshop on Consumer Disclaimers
The complexity of developing and delivering product disclosures to consumers was the subject of a day-long workshop hosted by the Federal Trade Commission (FTC) on...

The Story of Homeopathy Told in Upcoming Feature Documentary
The homeopathic community has no shortage of positive stories to tell—from the ways our drugs have improved the health of countless individuals, to the ways...
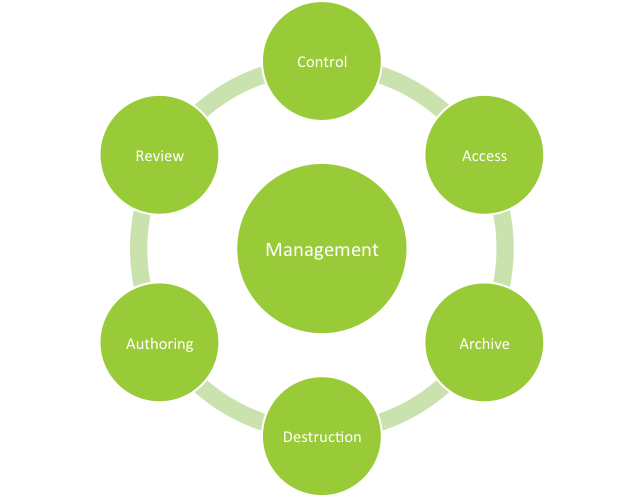
Document Management Systems
It is often said that the pharmaceutical industry produces two products: healthcare products and the paper that goes along with it. During the past 20...

What homeopathic manufacturers need to know, and what the FDA wants to see.
By Eric Foxman, AAHP Secretary Late last month, I joined Mark Vermette and Lyn Agostinelli of Halloran Consulting Group as they prepared for our upcoming...

Is Your Arnica Contaminated?
The American Botanical Council (ABC) has published their latest Adulteration Bulletin and it addresses our industry’s most widely used herb: Arnica. Adulteration of this source...

Only the Best Products Should Reach the Market
By Mark Land, AAHP President Many of today’s homeopathic products originated because of the contributions of our predecessors. These medicines were developed based on the...