Posts by Hayley Sotolotto
Pharmacy Spotlight: Don Summerfield of Medly Pharmacy
What are consumers looking for from homeopathy today? As manufacturers, how can we best serve them? An excellent way to get a pulse on our consumers is to look to…
Read MoreHomeopathic Promoters to Note FTC is Revising its Guide on Endorsements and Testimonials
By Mark Land, AAHP President Of the thousands of homeopathic products in the marketplace, few are ever seen advertised on television — historically, the mass medium to blanket the large…
Read MoreYour NDC Numbers are About to Change
By Eric Foxman, AAHP Secretary FDA has published a proposal that will change the required National Drug Code (NDC) format from 10 to 12 digits. Once finalized, all NDC holders…
Read MoreThis Year’s AAHP Summit On Hold Until 2023
Now in its fourth year, the AAHP Summit series has gathered the industry for feedback while the HPCUS works through five compliance gaps that challenge manufacturers and FDA inspectors alike.…
Read MoreFDA Seeks Accelerated Drug Approval, DSHEA, Cosmetics Legislative Reforms
By Pete Evich, AAHP Government Relations As part of the President’s FY 2023 Budget proposal, FDA has called upon Congress to take up legislation that would give the agency additional…
Read MoreThe Regulation of Homeopathic Medicines in 2022
By Mark Land, AAHP President The content in our June 2022 newsletter on regulation turned out quite different than I imagined when we discussed it at an editorial planning…
Read MoreRegulation of Homeopathic Medicines in Canada
Submitted by David Skinner, Canadian Homeopathic Pharmaceutical Association Homeopathic medicines (HMs) are regulated in Canada by Health Canada, the federal government department responsible for ensuring the safety, efficacy, and…
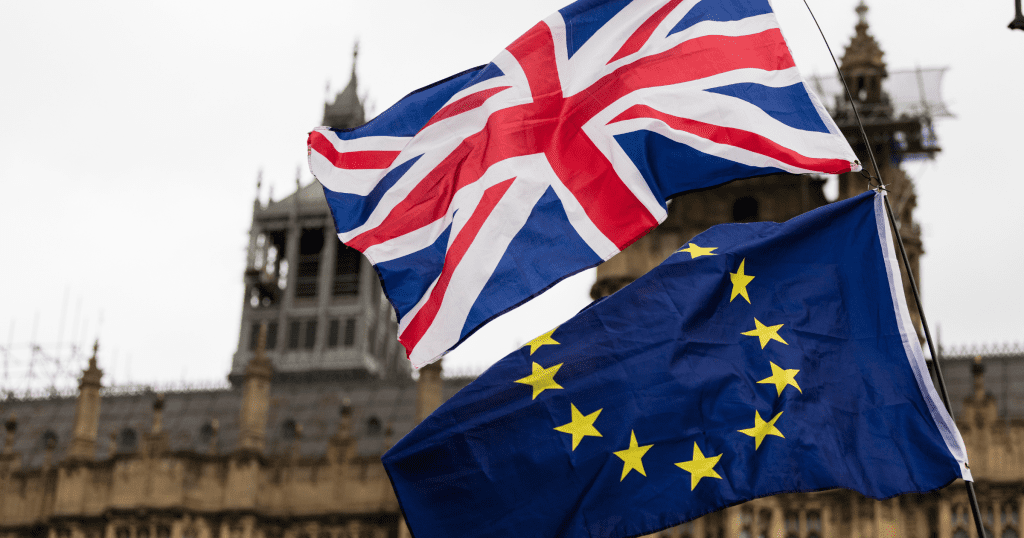
Read MoreRegulatory Status of Homeopathy in the EU & UK: What’s Happened Since Brexit?
By Steve Mann, Director of External Regulatory Affairs, Nelsons To say the world has changed over the last couple of years might just be the understatement of the century…
Read MoreA Global Perspective on Effectiveness Standards for Homeopathic Medicinal Products: Is there a “Third Way”?
By Robbert van Haselen, Chair, HPCUS Clinical Documentation Committee; Director, World Integrated Medicine Forum We currently live in an increasingly polarized world, and this is also reflected in the…
Read MoreRecent HPCUS Meetings and Updates
By Eric Foxman, Pharm. (Ret.), AAHP Secretary The Homeopathic Pharmacopoeia Convention of the United States held its annual meeting in the latter half of April. This included both the Board…
Read More