Non-Application Product Statistics in FDA’s Office of Pharmaceutical Quality Annual Report

For the sixth time, the Office of Pharmaceutical Quality (OPQ) in FDA’s Center for Drug Evaluation and Research (CDER) published its “Fiscal Year 2023 Report on the State of Pharmaceutical Quality.” The June 12 report contains three statistics for homeopathic products and manufacturing sites.
- Manufacturing Site Demographics (page 2)
“At the end of FY2023, the CDER Site Catalog included 4,819 drug manufacturing sites (Table 1), which represents a 14% increase in the number of sites over the past five years. Of all FY2023 drug manufacturing sites, 40% are in the “No Application” sector, indicating that all products manufactured at those sites are marketed in the U.S. without approved FDA applications. The majority of this sector includes over-the-counter (OTC) monograph products, but also includes marketed unapproved prescription drug products and homeopathic products. The remaining 60% of sites manufacture at least one application product.” - Drug Product Demographics (page 5)
“The CDER Product Catalog is a regularly updated record of all application products (NDAs, ANDAs, and BLAs) and non-application products (OTC monograph products, marketed unapproved prescription drugs, homeopathic products, etc.). At the end of FY2023, the Product Catalog included 13,572 ANDAs, 3,593 NDAs, 354 BLAs, and 131,367 non-application product NDCs. Some of these applications may include multiple NDCs, each with different strengths, concentrations, or package configurations. In FY2023, the number of applications in the catalog increased for all categories. The largest percent increase was observed for BLA products (9%) and the smallest increase was for NDA products (1.5%). Overall, the number of products listed increased 6% to 148,886 in FY2023.” - Recalls (page 15)
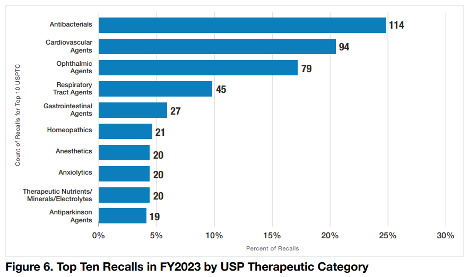
In FY2023, there were 674 drug products recalled, down 26% from the high of 912 in FY2022. Although only 21 (3.1%) of these products were homeopathic, it was enough to propel the category mid-way up a chart of “Top 10 Recalls in FY2023 by USP Therapeutic Category.” Antibacterials had the highest percentage of recalls with 114 products. Antiparkinson agents were last on the “top 10” list with 19 recalled products. In general the section on recalls focused on ophthalmics.