The FDA Adverse Event Reporting System (FAERS)

By Kristina Skowronek, Dir. of Quality and Regulatory Compliance, Boiron, Inc.
The FDA Adverse Event Reporting System (FAERS) has a public dashboard online where healthcare practitioners, retailers, consumers, and industry can search for adverse event case information for pharmaceutical products. The Food and Drug Administration (FDA) developed the dashboard to increase transparency for product safety reports and encourage participation in adverse event reporting to the agency as drug product safety surveillance is critical to protecting public health.
When one opens the dashboard via the website, there is a disclaimer to acknowledge that the viewable data is only part of the total safety data submitted to FDA and these data do not constitute a causal relationship between the product and the reported event. The data initially appearing on the dashboard is high level, but it is very interactive based upon the data you’d like to see. If you are looking to see a full case report or set of reports, a Freedom of Information Act request can be submitted to FDA to obtain a copy of individual case reports: https://www.fda.gov/regulatory-information/freedom-information/how-make-foia-request
On the primary page, there is a table showing Total Reports in descending order by year with a breakdown of mandatory expedited, mandatory non-expedited, direct and Biologic Safety Reports. Mandatory reports are provided by manufacturers based upon serious and unexpected adverse events not described in the product’s labeling or events which are both serious and expected based on product labeling. Direct reports are submitted directly to FDA by consumers or healthcare practitioners. The page is highly interactive, and you can view a chart by year and report type. If you zoom in or out on the page, you can either expand or collapse the high-level data shown.

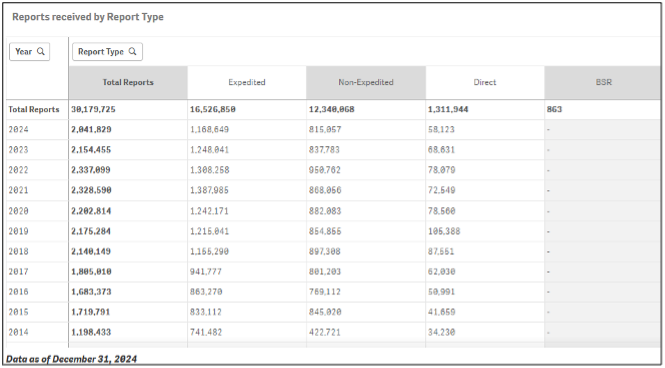
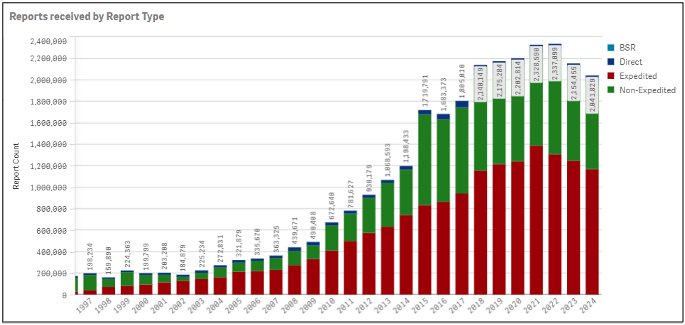 Data Elements can be expanded, exported, or shared by right-clicking on the specific data. The data can be viewed by report type, reporter type (i.e., consumer or health care professional), region, seriousness, patient age or gender. There is also a search function to view data by specific product or adverse event reaction term. If a search by product is conducted, you can choose an individual item from a drop down which appears and the data for the chosen product will be displayed in number of cases, year reported, and percent of total cases for the item. Data are generally published quarterly so if you seek data from Q1 of 2025, you can check FAERS in April 2025.
Data Elements can be expanded, exported, or shared by right-clicking on the specific data. The data can be viewed by report type, reporter type (i.e., consumer or health care professional), region, seriousness, patient age or gender. There is also a search function to view data by specific product or adverse event reaction term. If a search by product is conducted, you can choose an individual item from a drop down which appears and the data for the chosen product will be displayed in number of cases, year reported, and percent of total cases for the item. Data are generally published quarterly so if you seek data from Q1 of 2025, you can check FAERS in April 2025.
To examine safety data on products which may not have been reported directly to the manufacturer, the FAERS dashboard can be a valuable tool. Case report information can be extracted from the system routinely and analyzed for important trends about patient experiences and outcomes of which companies would not otherwise be aware. The individual product search shows total number of cases reported to FDA during a particular year, but you can also drill down to the adverse event terms referenced to see specifically what medical events were reported.
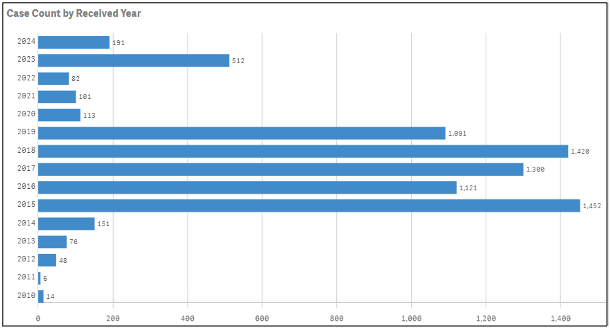
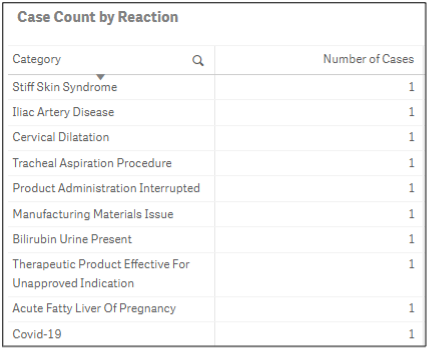
Reaction terms show what medical events have been reported as well as if a product was used for an off-label or unapproved indication. A comparison to product labeling can be done to determine if label revisions are needed to communicate more clearly to consumers what the product is indicated for, directions for use, or necessary warnings.
FDA requirements and guidance are clear in their expectations of industry diligence in product safety and adverse event reporting not only for traditional allopathic pharmaceutical preparations, but also for homeopathic products. Case reports meeting the definition of a serious adverse event are subject to reporting to FDA and are classified as mandatory whether they are expected or not. Product labeling must include contact information for consumers to inquire about products or report adverse experiences, but companies are also responsible for monitoring other possible sources of safety reports on their products such as literature, company social media sites, and company websites. If a company chooses to examine retail websites or product reviews, identified adverse events are to be registered and reported to FDA in accordance with the regulations and applicable guidance.
The FAERS dashboard provides another resource homeopathic pharmaceutical companies can use to examine product safety and protect the public health. FDA has recently expanded its own use of FAERS in a data mining study searching for adverse events reported among Amazon product reviews: Using natural language processing to characterize and predict homeopathic product-associated adverse events in consumer reviews: comparison to reports to FDA Adverse Event Reporting System (FAERS).1
The study showed that the Amazon data found was in line with the data in FAERS and in both places, FDA could gain a solid understanding of consumer perceptions and usage of homeopathic products. The article notes FDA looks to expand their usage of these data analysis methods therefore, it would be in the best interest of homeopathic companies to familiarize themselves with the methods and the data to remain compliant in their pharmacovigilance activities and to grow their surveillance programs with the times. Read more from AAHP on this study here: https://theaahp.org/newsletter/fda-and-univ-of-al-analyzed-amazon-consumer-reviews-for-homeopathy-adverse-events/
References
1. Konkel K, Oner N, Ahmed A, Jones SC, Berner ES, Zengul FD. Using natural language processing to characterize and predict homeopathic product-associated adverse events in consumer reviews: comparison to reports to FDA Adverse Event Reporting System (FAERS). J Am Med Inform Assoc. 2023 Dec 22;31(1):70-78. doi: 10.1093/jamia/ocad197. Erratum in: J Am Med Inform Assoc. 2024 Jan 18;31(2):548. doi: 10.1093/jamia/ocad225. PMID: 37847653; PMCID: PMC10746310. https://pubmed.ncbi.nlm.nih.gov/37847653/
