All
- All
- Articles
- Calendar
- Compliance
- Newsletter
- Position Statements
- Press Release
- Regulatory
- Uncategorized

NCH First in AAHP Collaboration Effort
Dr. Lauri Grossman, President of the Board of Directors, National Center for Homeopathy (NCH) Last autumn, the American Association of Homeopathic...

How You Can Help the State of Research for Homeopathy
By Mark Land, AAHP President Rachel reported that one facet of the research database included slightly more than 220 studies, covering just over...

117th Congress and Biden Administration Underway
By Pete Evich, AAHP Government Affairs On Jan. 20, Joe Biden was sworn in as the 46th President of the United States. On the...

Your Reputation Precedes You
Brian Westerlind, Editor of the Network News, writes in his article on warning letters: "Each day AAHP works to promote excellence in the practice...

4 Simple Ways to Keep Your Homeopathic Manufacturing Process Safe and Effective
Each day AAHP works to promote excellence in the practice of homeopathic manufacturing. While achieving true excellence is a lofty goal, it begins with a...
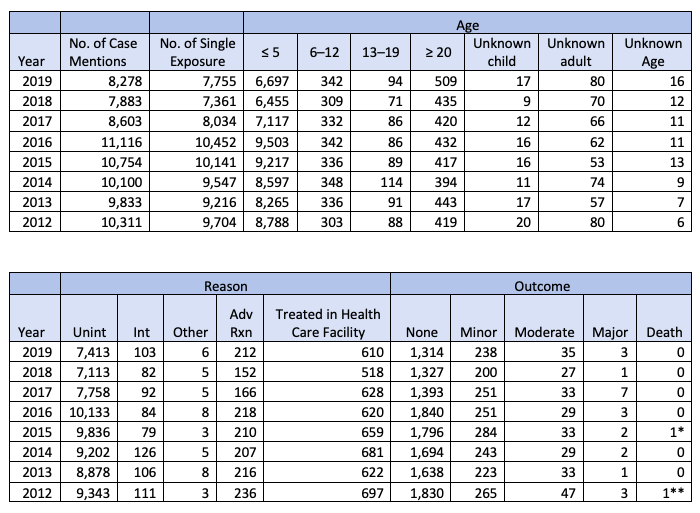
Poison Control Center Statistics on Homeopathy
What's Your Company's Safety Statistics? When FDA announced its public hearing on homeopathy, the agency cited 10,311 reported poison exposure cases related to "homeopathic...

Homeopathy e-Reputation Index Analysis
May 2016-September 2020 (Source: Brandwatch Service/Vanksen Agency) by Alissa Gould, Dir., Corp Comm. & Public Affairs, Boiron USA Reviewing the e-reputation for homeopathy in...
