All
- All
- Articles
- Calendar
- Compliance
- Newsletter
- Position Statements
- Press Release
- Regulatory
- Uncategorized

What homeopathic manufacturers need to know, and what the FDA wants to see.
By Eric Foxman, AAHP Secretary Late last month, I joined Mark Vermette and Lyn Agostinelli of Halloran Consulting Group as they prepared for our upcoming...

Is Your Arnica Contaminated?
The American Botanical Council (ABC) has published their latest Adulteration Bulletin and it addresses our industry’s most widely used herb: Arnica. Adulteration of this source...

Only the Best Products Should Reach the Market
By Mark Land, AAHP President Many of today’s homeopathic products originated because of the contributions of our predecessors. These medicines were developed based on the...

Homeopathic Association Works with FDA to Educate Manufacturers
MILWAUKIE, Ore. (July 6, 2016) – The American Association of Homeopathic Pharmacists’ (AAHP) latest educational webinar illustrates the homeopathic industry fostering a relationship with the...
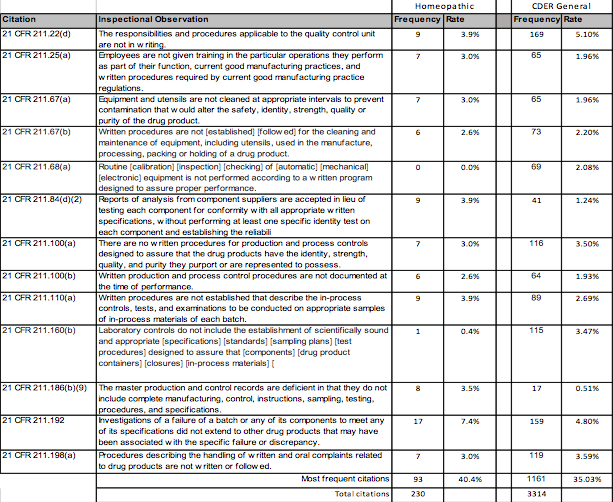
US FDA Establishment Inspections: Outcomes for Labelers for Homeopathic Drugs
By Mark Land, AAHP President, June 2016 Abstract Labelers of homeopathic drugs are a subset of drug manufacturing establishments located within the United States and...

Understanding OTC Monograph Reform: Where Do We Stand Now?
By J.P. Borneman, Ph.D. and Alvin J. Lorman, J.D. The FDA’s Over-the-Counter Drug Review (OTC Review) has always been important to homeopathy, despite the fact...

Adulterants in Your Botanical Source Materials – How Do You Know?
By Eric L. Foxman, R.Ph Many commercially available botanical products have been adulterated prior to their introduction to the market. This is a problem that...
