All
- All
- Articles
- Calendar
- Compliance
- Newsletter
- Position Statements
- Press Release
- Regulatory
- Uncategorized

Outlook 2017
By Mark Land, AAHP President March 1, 2017 2017 is shaping up to be a year in motion—both for our industry and our country. With...

The FTC and the Web
By Alvin J. Lorman, Association Counsel March 1, 2017 The recent claim by the Federal Trade Commission (FTC) that its policy statement on marketing of...

Manufacturers of Homeopathic Medicines Review FDA Test Results and New Application of Technology
MILWAUKIE, Ore., Feb. 14, 2017 — The U.S. Food and Drug Administration (FDA) announced on Jan. 27 that its laboratory analysis found inconsistent levels of...

AAHP in 2016 and a Review of New Member Resources
Jan. 26, 2017 In 2016, the Education Committee produced four robust webinars, and industry leaders united at two membership meetings. As we step forward into...

The Tale of the Contract Manufacturer: A Story Based on an AAHP Member Question
By Eric Foxman, AAHP Secretary Once upon a time, a special creature, the CIAMP (Company Interested in Alternative Medical Products), wanted Phoxman Farmaceuticals (PhoxFarm) to...

Congress to Tackle “Must Pass” FDA Legislation Next Year
The Prescription Drug User Fee Act (PDUFA) authorizes the Food and DrugAdministration (FDA) to collect fees from Rx pharmaceutical companies to help fund the agency's...
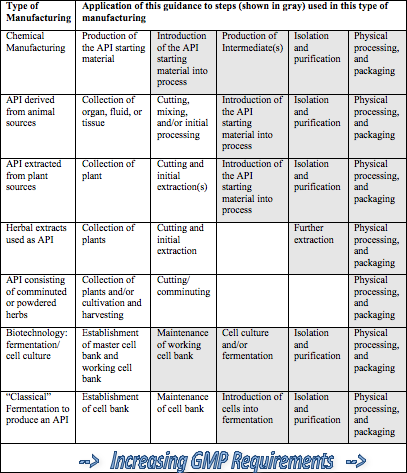
What About Your APIs and Q7 Requirements?
By Eric L. Foxman, R.Ph. Jan. 26, 2017 In September 2016, the U.S. Food and Drug Administration (FDA) issued a guidance document, entitled Q7 Good...