All
- All
- Articles
- Calendar
- Compliance
- Newsletter
- Position Statements
- Press Release
- Regulatory
- Uncategorized

Protected: Virtual Summit Presentations
This content is password-protected. To view it, please enter the password below. Password:
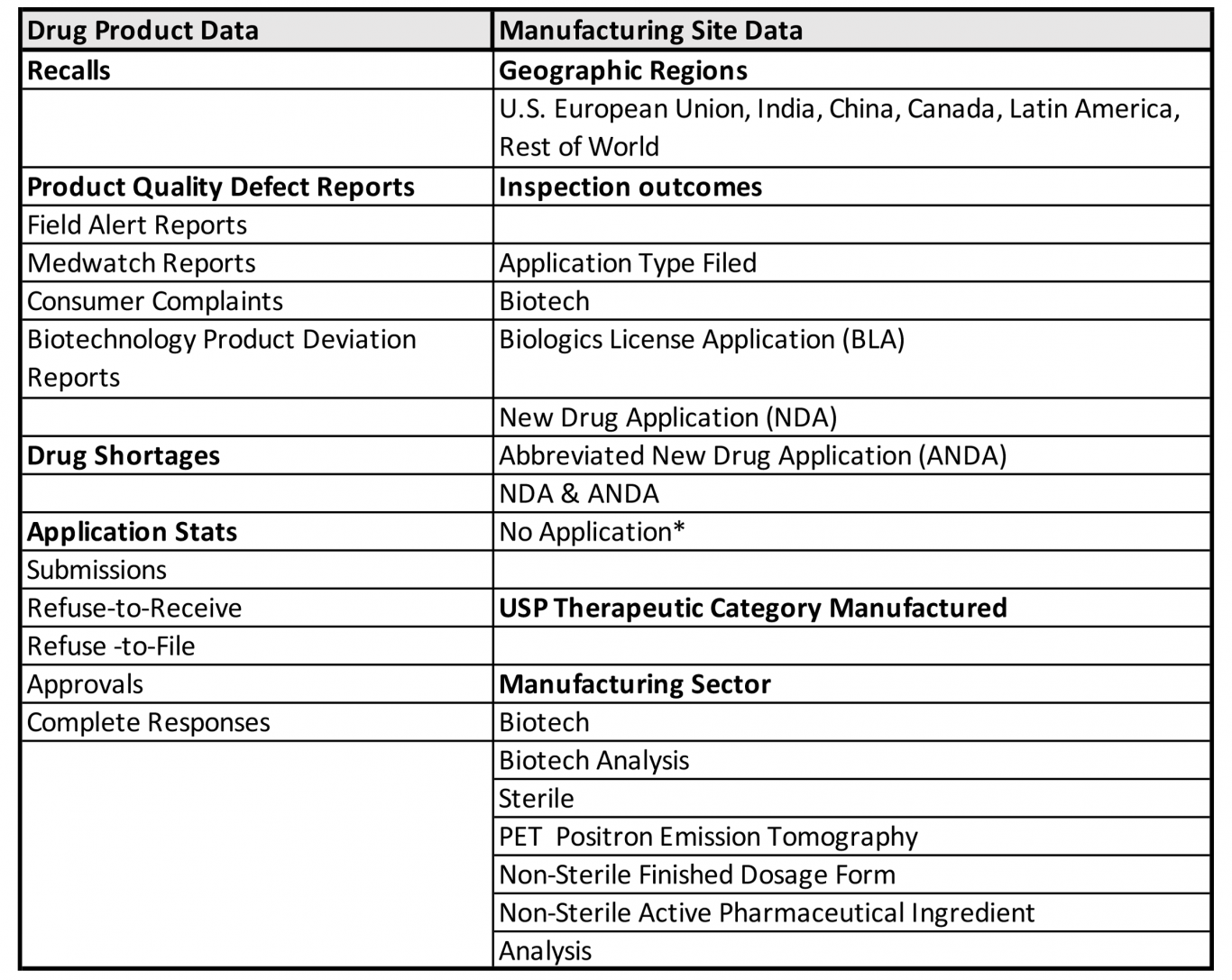
New FDA Report on the State of Pharmaceutical Quality
By Mark Land, AAHP President A quality drug is consistently safe, effective, and free of contamination or defects. Patients and consumers expect quality drugs with...

Notes on the Future: AAHP Outlook 2020
AAHP’s Board of Directors just completed its fourth annual strategy retreat. Together we reflected on the accomplishments, challenges, and opportunities we’ve had during this truly...

AAHP Reinforces MediNatura’s Defense Against FDA Action
In support of our member company MediNatura, Inc. challenging FDA’s attack on its prescription injectable homeopathic products, AAHP filed an amicus brief in the United...

Key Findings from AAHP’s Homeopathic Shopper Report & Panel
To help homeopathic manufacturers and marketers best serve consumers during this unusual time in history, AAHP surveyed nearly 600 dedicated homeopathy users. The results might...

Sourcing Homeopathic Active Pharmaceutical Ingredients: Controls, Arrangements
By Mark Land, AAHP President In light of recent warning letters to homeopathic manufacturers, I have received several questions regarding procurement of homeopathic active pharmaceutical...

2019-2020 HPUS Changes: What Homeopathic Companies Need to Know
The AAHP hosted a webinar in June on Homeopathic Pharmacopoeia Updates 2019-2020: Essential Information Companies Need to Know. During the course of this concise webinar,...
