All
- All
- Articles
- Calendar
- Compliance
- Newsletter
- Position Statements
- Press Release
- Regulatory
- Uncategorized

How to Use Ignatia amara
(Image: Steven Edward/Flickr) By Todd A. Hoover, MD, DHt The homeopathic medicine Ignatia amara is derived from the St. Ignatius Bean (from the Strychnos ignatii...

Trade Shows That Help You Break Into Mass Market
By Ray Petrick, Vice President of Sales, Boiron USA Sales of homeopathic products in the U.S. totaled $1,354 billion in 2017, according to the annual...
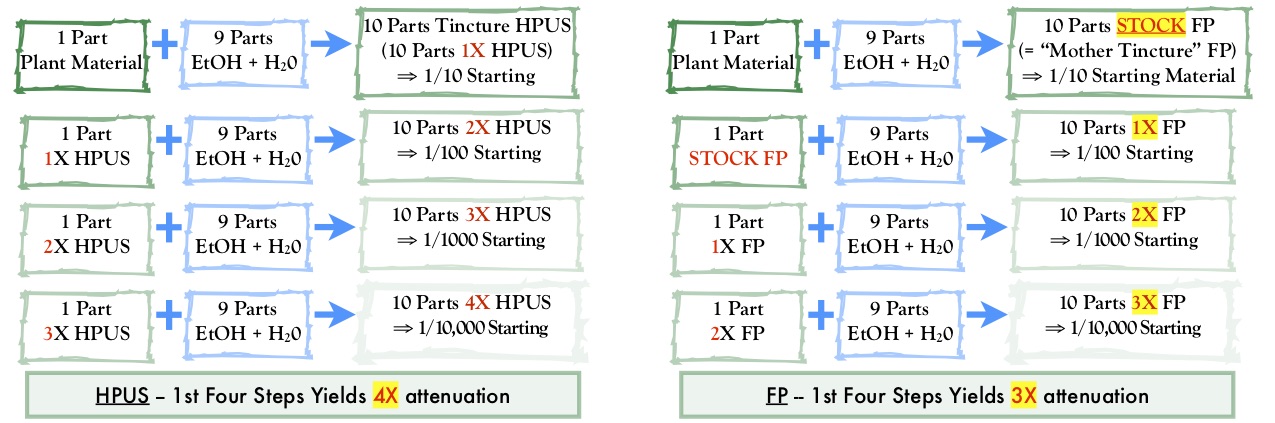
The French Pharmacopeia
By Eric Foxman, R.Ph., AAHP Secretary A quick look at its structure and contents as well as two key considerations for potential adulteration and misbranding...

Homeopathic Medicines at Retail
By Mark Land, AAHP President Every year or so, I survey my local CVS store for the number and type of homeopathic drug products on...

How to Use Gelsemium sempervirens
By Todd A. Hoover, MD, DHt Gelsemium sempervirens is a plant substance, commonly known as yellow jasmine, native to tropical and subtropical Americas. The traditional...

Homeopathy, A Niche No More
The following is an excerpt taken from an article that appeared in the September issue of Drug Store News (DSN) and potentially read by 33,000...